 Noninvasive Prenatal testing (NIPT)
Noninvasive Prenatal testing (NIPT)
Test Overview
NIPT is a screening test to detect fetal chromosomal aneuploidies (Down Syndrome, Edward Syndrome and Patau Syndrome) using cell free DNA (cfDNA) from maternal blood. During pregnancy fetal cfDNA is released by the placenta into the mother’s bloodstream.
Test Overview
NIPT is a screening test to detect fetal chromosomal aneuploidies (Down Syndrome, Edward Syndrome and Patau Syndrome) using cell free DNA (cfDNA) from maternal blood. During pregnancy fetal cfDNA is released by the placenta into the mother’s bloodstream.
The maternal blood contains mixture of both maternal and fetal cfDNA derived from apoptotic cells. By using Next Generation Sequencing (NGS) the total cfDNA in maternal plasma is deeply sequenced and the relative contribution from the mother and fetus is accurately estimated.
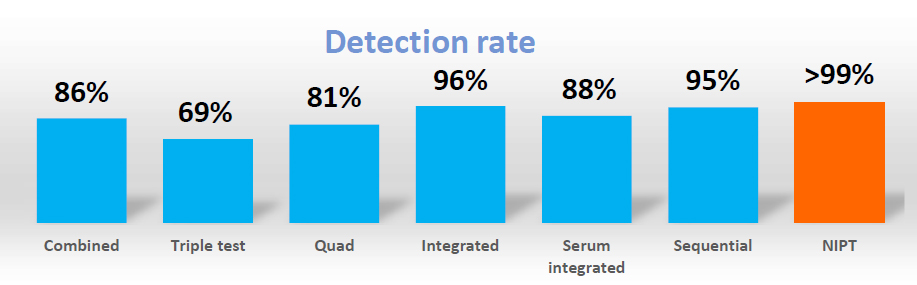
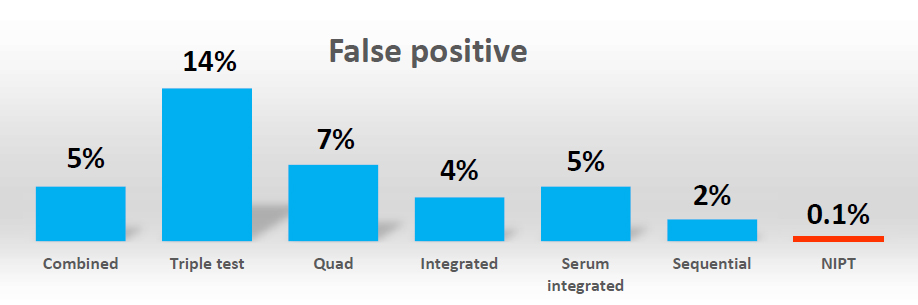
NIPT offers higher sensitivity and specificity than current screening test which are based on phenotypic factors (e.g. Integrated Prenatal Screening or First Trimester Screening). It has a lower false positive higher detection rate meaning fewer people will need to have follow-up invasive testing which is associated with a risk of pregnancy loss. The American College of Obstetrician and Gynecologists (ACOG) and International Society of Prenatal Diagnosis (ISPD) have stated that NIPT should be offered as a screening option to all pregnant women regardless of maternal age and with a positive serum screening test result.
Reason for referral- Advanced maternal age (>35 years)
- Previous baby with chromosomal aneuploidy
- Positive serum maternal screening test
- History of miscarriage
- Pregnancy through IVF treatment
Analysis method
Maternal blood is collected in a specialized tube (STRECK TUBE) that prevents the cells from breaking down and protecting the cfDNA. Cell free DNA is then extracted from the plasma and prepared for sequencing. By using next generation sequencing technology (NGS) the total cfDNA from maternal plasma is deeply sequenced. The relative contribution from the mother and fetus is accurately estimated by bioinformatic analysis.
Sample requirement
10.0 mL maternal blood collected in STRECK tube. If the sample is to be collected outside please contact the lab for support. Samples collected in ordinary tube cannot be used for this particular test.
Preparation
Blood samples from women who are at least 10 weeks of pregnant.
Instrument platform
Ion GeneStudio S5, Next Generation Sequencing Platform (Thermo Fisher Scientific, USA).
